The great resources of the iron ore and the vast resources of manpower and energy have kept manufacturers’ attention focusing on using sponge iron instead of scrap iron. In the direct reduction method, it has been tried for the iron ore oxygen to be almost completely removed before melting. Considering the fact that the resulted sponge iron contains some of the worthless rock (gangue) as well as other impurities, the chemical composition of sponge iron is one of the main factors affecting the quality of this material which determines its metallization percentage.
Generally, when using sponge iron, some factors increase energy consumption. Percentage of metallization in energy consumption during the process of melting and producing steel is so effective. If the metallization is low, the FeO level will rise and this will cause an increase in energy consumption. One of the reasons of high FeO increases energy can be increased consumption of coke, lime, Dolomite, melting time, slag volume and FeO reduction being endothermic.
(FeO)+(C)=[Fe]+{CO}
On the other hand, the FeO increases refractory corrosion, reduces melting efficiency and also increases electrode corrosion in electrical arc furnaces.
Of the harmful impurities of sponge iron, SiO2, S, P, and Al2O3 can be noted of which the excessive presence causes an increase in energy and refractory corrosion. Increasing SiO2 in sponge iron reduces slag alkalinity and increases refractory corrosion, so more lime should be charged to maintain slag alkalinity. This, in return, will increase energy.
The energy in EAFs can be supplied in two ways:
- Electrical energy, as the source of energy in EAFs, accounts for about 60% of the required energy.
- Chemical energy acts out through either metallurgical reactions such as oxidation reactions of the elements or secondary combustion reactions designed to increase the share of chemical energy and decrease losses in the supply of required heat.
Hence, carbon plays an important role in this regard. Carbon is considered in three parts as an inevitable effective factor in reducing the electrical energy of EAFs:
- The reaction of carbon with oxygen in the furnace releases a great deal of energy.
- Foamy Slag: the creation of foamy slag is one of the most economical and at the same time most useful factors in reducing energy and refractory consumption.
- Secondary combustion: this burns CO on melt surface and increases furnace efficiency.
After metallization, one of the most important elements in sponge iron is carbon content. The presence of carbon in the sponge iron eliminates impurities from coke injection such as ash, sulfur, and volatiles.
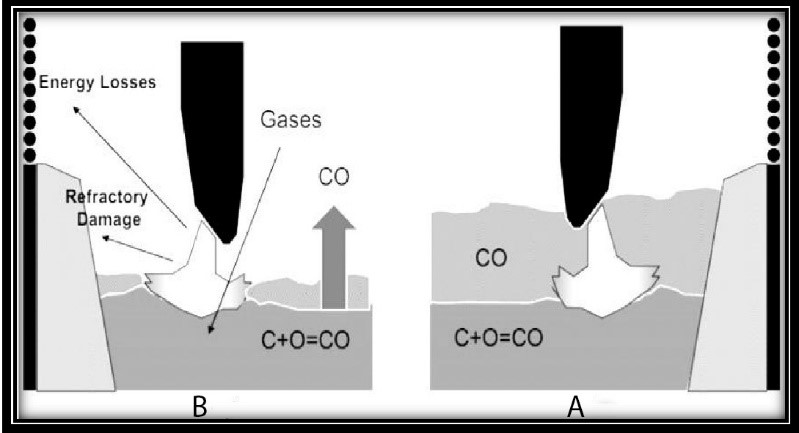
If there is no limitation to the supply of required oxygen, the energy consumption will be reduced by about 5kWh through charging with high-carbon sponge iron. As shown in below Fig., a foamy slag, acts as an arc cover, causing the arc length to increase, energy loss to decrease and also the furnace refractory to be protected. However, if there is no foamy slag, the condition is quite the opposite. Reducing electrode consumption, melting time, and dissolving gases in the steel are other advantages of foamy slag.
Using sponge iron and replacing it with scrap has the following benefits (to name a few):
- Achievement to the required analysis due to the consistency of the chemical composition and the low content of the gangue.
- Ease of storage, the possibility of continuous charging of sponge iron, easy slagging, ease of control of smelting operations and lack of power oscillation.
- Improving consumption of energy, raw material and refractory.
- Reducing electrode failure, hazards, and contaminants.
Result
The chemical composition of sponge iron has a significant impact on the energy and refractory consumption of EAFs. Quality of sponge iron is determined based on its Metallization and residual oxygen. The higher metallization and the lower FeO cause the higher free iron content in the sponge iron. Of course, the presence of carbon, which is in the form of Fe3C, is important. Carbon reduces the consumption of refractory, energy, electrodes and the slag by combining with oxygen, creating foamy slag and exothermic reaction.


